Particulate nature of matter (IGCSE)
Core
•State the distinguishing properties of solids, liquids and gases
• Describe the structure of solids, liquids and gases in terms of particle separation, arrangement and types of motion
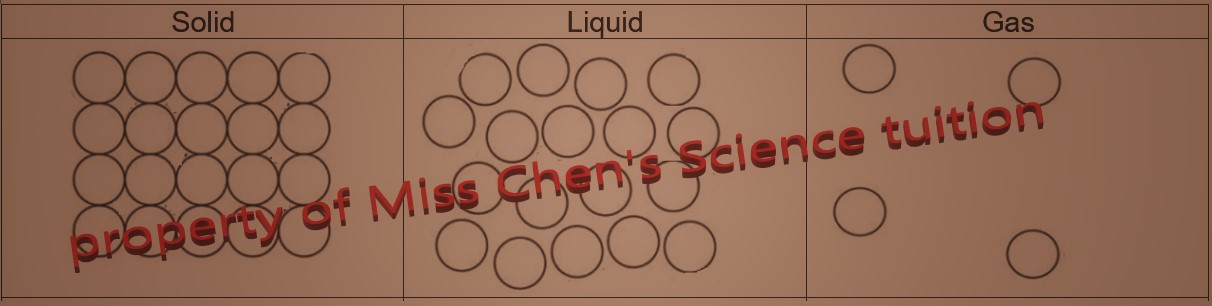
| Solid |
Liquid |
Gas |
| Solids have a fixed volume and fixed shape. Solids cannot be compressed. |
liquids take up the shape of their container but have a fixed volume. Liquids cannot be compressed. |
Gases have no fixed volume and shape. Gases can be compressed. |
| Very strong forces of attraction between particles |
Weaker forces of attraction forces than solids |
Very weak, almost no forces of attraction
|
| Particles are very closely packed and are in regular arrangement. They vibrate in fixed positions, |
Particles are close together but in disordered arrangement. They slide past each other. |
Particles are very far apart from each other and are in random order. They move freely and very quickly.
|
- Describe changes of state in terms of melting, boiling, evaporation, freezing, condensation and sublimation
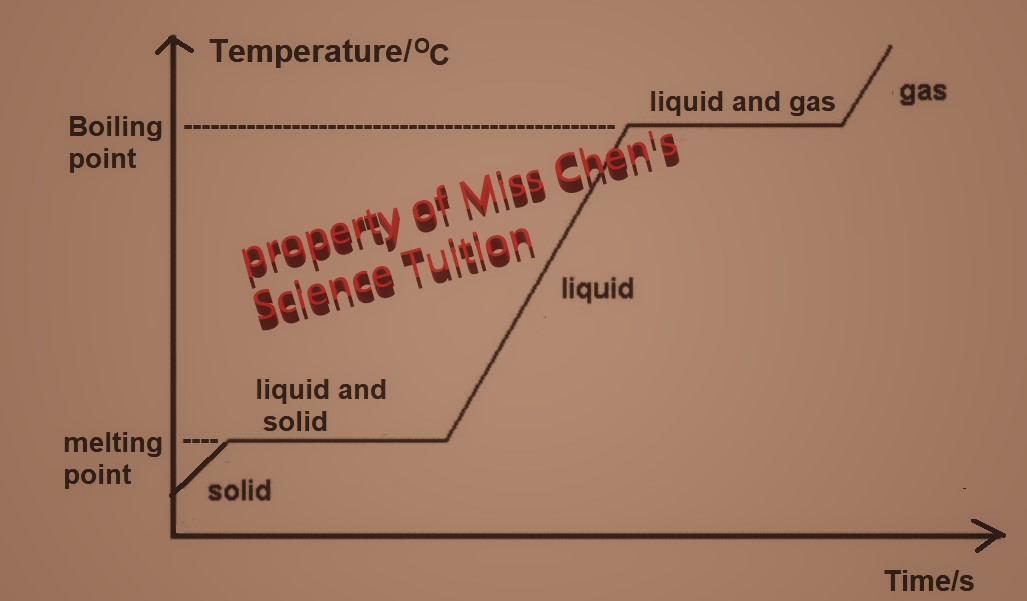
| Changes of States |
Name of the Physical Process |
| Solid to liquid |
Melting |
| Liquid to gas |
Boiling / evaporation |
| Solid to gas |
Sublimation |
| Gas to liquid |
Condensation |
| Gas to solid |
Deposition |
| Liquid to solid |
Freezing |
• Describe qualitatively the pressure and temperature of a gas in terms of the motion of its particles
As the temperature of gas increases, the particles have more kinetic energy and move more quickly.
As the temperature falls, the particles move slower.
Gas pressure is caused by the force exerted on walls of container when the gas particles collide against the walls of the container. As the temperature of the gas increases, the particles gain more kinetic energy and they move more quickly and collide against the walls more and force exerted is more. Pressure increases.
When a gas is compressed into a smaller space, its pressure increases. If enough force is applied, the particles can be pushed so close that the gas turns into a liquid.
• Show an understanding of the random motion of particles in a suspension (sometimes known as Brownian motion) as evidence for the kinetic particle (atoms, molecules or ions) model of matter
In liquids: potassium manganate (VII) in a beaker of water. The purple crystals dissolve, purple colour spreads and turn water purple.
In gases: Place an open gas jar of air upside down on an open gas jar containing a few drops of red-brown bromine. The reddish-brown colour spreads upwards
• Describe and explain diffusion
Diffusion is the spreading of a substance from a region of high concentration to a region of low concentration due to the random collisions of particles. and bouncing off in all directions till the particles are evenly spread out.
Supplement
- Explain changes of state in terms of the kinetic theory
Melting: Heating a solid cause the particles to gain more kinetic energy making them vibrate faster and more, making the solid expand. When they gain enough energy to break the strong forces of attraction that hold them in solid state and the particles start to move and slide past one another.
Boiling: When a liquid is heated, its particles gain more kinetic energy and move faster. They bump into each other more and bounce further apart. This makes the liquid expand. At the boiling point, the particles gain enough energy to overcome the strong forces that hold them in the liquid state. Particles escape the liquids surface and move freely in continuous rapid motion.
Sublimation: Heating a solid cause the particles to gain more kinetic energy making them vibrate faster and more, making the solid expand. When they gain enough energy to break the strong forces of attraction that hold them in solid state and the particles start to move freely in continuous rapid motion.
During changing of state (melting, boiling, sublimation), temperature is constant as any heat energy supplied is used to overcome the strong forces of attraction and not to increase kinetic energy of the particles.
Condensation: When a gas is cooled, the particles lose kinetic energy. They bump into each other less and bounce less. This makes the gas contracts. the particles lose enough energy, forces of attraction are strong enough to hold them in the liquid state. They become closer and slide past each other.
Freezing: : When a liquid is cooled, the particles lose kinetic energy. They become closer and move less. This makes the liquid contracts. When the particles lose enough energy, forces of attraction are strong enough to hold them in solid state. They become closer and vibrate in fixed positions.
• Describe and explain Brownian motion in terms of random molecular bombardment
It is the constant random movement of tiny particles suspended in a fluid which is caused by collision with fluid particles that are in continuous and random motion.
• State evidence for Brownian motion
Pollen grains are moving randomly in water and smoke particles moving randomly in air. Pollen grains and smoke particles are constantly bombarded by water molecules and air particles respectively.
• Describe and explain dependence of rate of diffusion on molecular mass
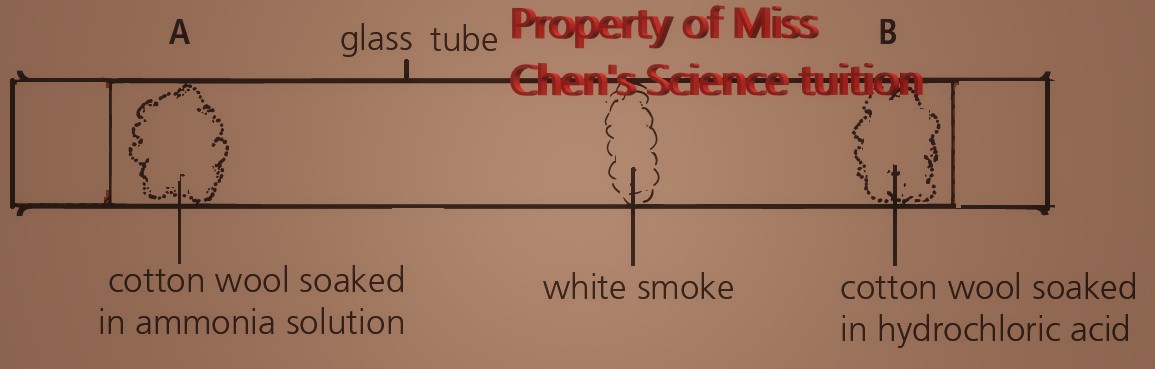
The lower the mass of its particles, the faster a gas will diffuse.
Ammonia solution and hydrochloric acid will evaporate to become ammonia gas and hydrogen chloride respectively.
Colourless gases, ammonia and hydrogen chloride, diffuse down the tube and react to form white solid of ammonium chloride.
NH3(g) + HCl(g) ⇌ NH4Cl(s)
Mr (NH3) = (14 x1) + (1 x 3) = 17, Mr (HCl) = (1 x 1) + (35.5 x 1) = 36.5
The white smoke forms closer to B as the ammonia molecules with lower relative molecular mass of 17 will diffuse faster and move further than the hydrogen chloride molecules with relative molecular mass of 36.5.