Covalent Bonding (IGCSE)
Molecules and Covalent bonds
Core
• Describe the formation of single covalent bonds in H2, Cl2, H2O, CH4, NH3 and HCl as the sharing of pairs of electrons leading to the noble gas configuration.
A covalent bond is formed when atoms share electrons.
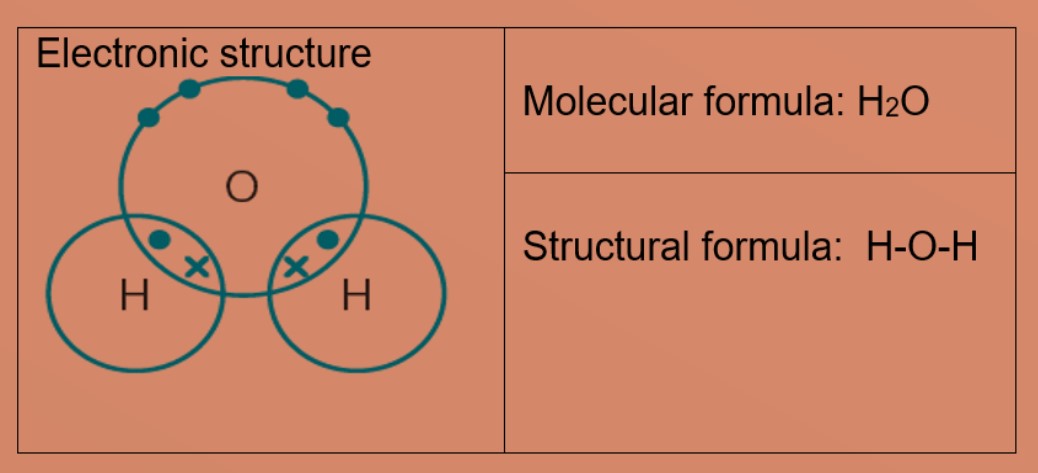
Eg 1: Hydrogen
A hydrogen atom has only one electron in the first shell. Each hydrogen atom needs one more electron to be stable. Two hydrogen atoms can share one electron with each other to achieve stable electronic configuration for the first electron shell.
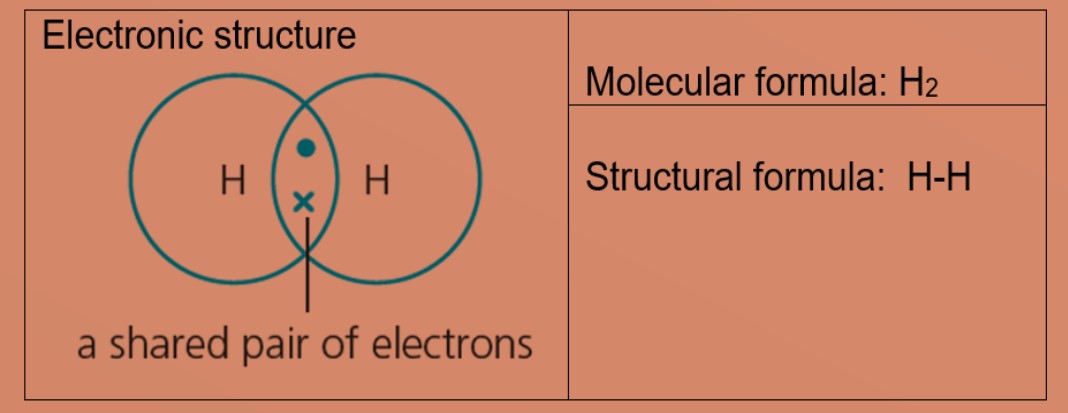
Eg 2: Chlorine, molecular formula: Cl2
A chlorine atom has 7 valence electrons. Each chlorine atom needs one more electron to be stable. Two chlorine atoms can share one electron with each other to achieve stable electronic configuration for the valence electron shell.
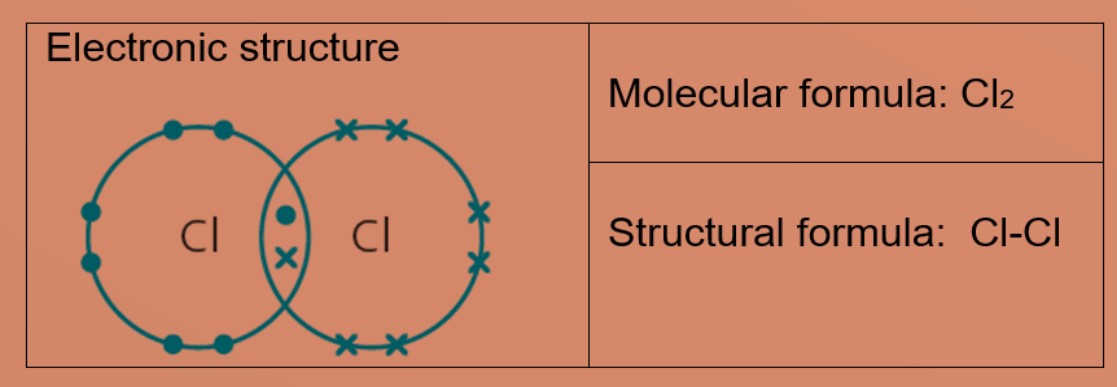
Eg 3 : Hydrogen chloride
Each chlorine and hydrogen atom need one more electron to be stable. The chlorine atom and hydrogen atom share one electron with each other. Chlorine and hydrogen then will have a stable electronic configuration of 2.8.8 and 2 respectively.
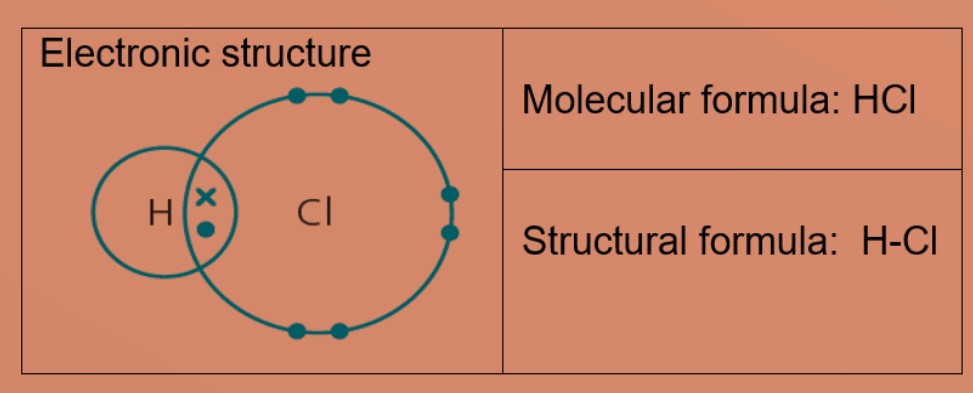
Eg 4 : Water
Oxygen atom needs two more electrons and hydrogen atom need one more electron to be stable. The oxygen atom shares 2 valence electrons with the two hydrogen atoms. Each hydrogen atom shares one electron with oxygen atom. Oxygen and hydrogen have a stable electronic configuration of 2.8 and 2 respectively.
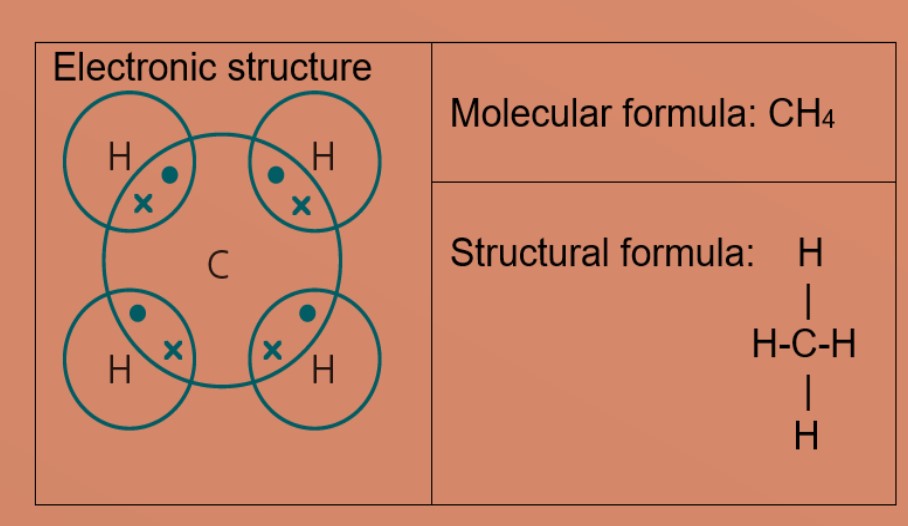
Eg 4: Methane
Carbon atom needs 4 more electrons to be stable. The carbon atom shares 4 valence electrons with four hydrogen atoms. Each hydrogen atom shares one electron with carbon atom. Carbon and hydrogen have a stable electronic configuration of 2.8 and 2 respectively.
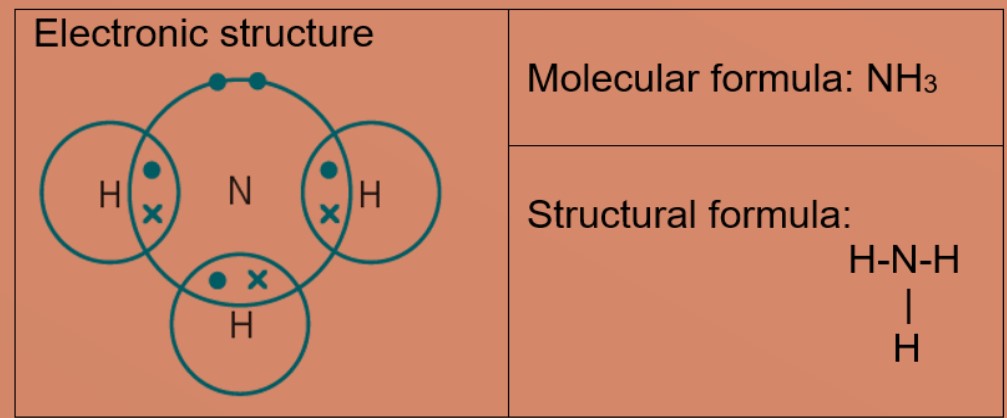
Eg 5: Ammonia
Nitrogen atom needs 3 electron more to be stable. Nitrogen atom shares 3 valence electrons with three hydrogen atoms. Each hydrogen atom shares one electron with nitrogen atom. nitrogen and hydrogen have a stable electronic configuration of 2.8 and 2 respectively.
• Describe the differences in volatility, solubility and electrical conductivity between ionic and covalent compounds
| Ionic compound |
Covalent compounds |
| Particles are ions |
Particles are molecules |
| High melting and boiling points due Giant ionic lattice structure with strong ionic bonds. Large amount of energy is needed to break these bonds. |
Low melting and boiling points due to Simple molecular structure with weak intermolecular forces. Small amount of energy is needed to overcome these forces. |
| Usually soluble in water, insoluble in organic solvents |
Usually insoluble in water, soluble in organic solvents |
| Conduct electricity in molten and aqueous state due to mobile ions, do not conduct in solid state |
Usually do not conduct electricity as there are no charged particles, no mobile ions or electrons |
Supplement
• Describe the electron arrangement in more complex covalent molecules such as N2, C2H4, CH3OH and CO2
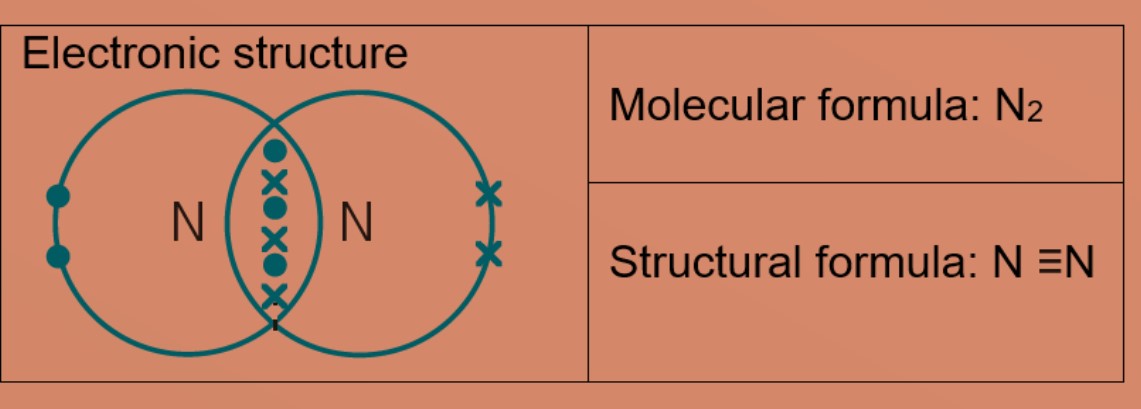
Eg 1: Nitrogen
A nitrogen atom has five valence electrons, it needs three more electrons to be stable.
Two nitrogen atoms share three electrons each, forming triple covalent bonds in the molecule with the formula N2. Each atom now has eight valence electrons and is stable.
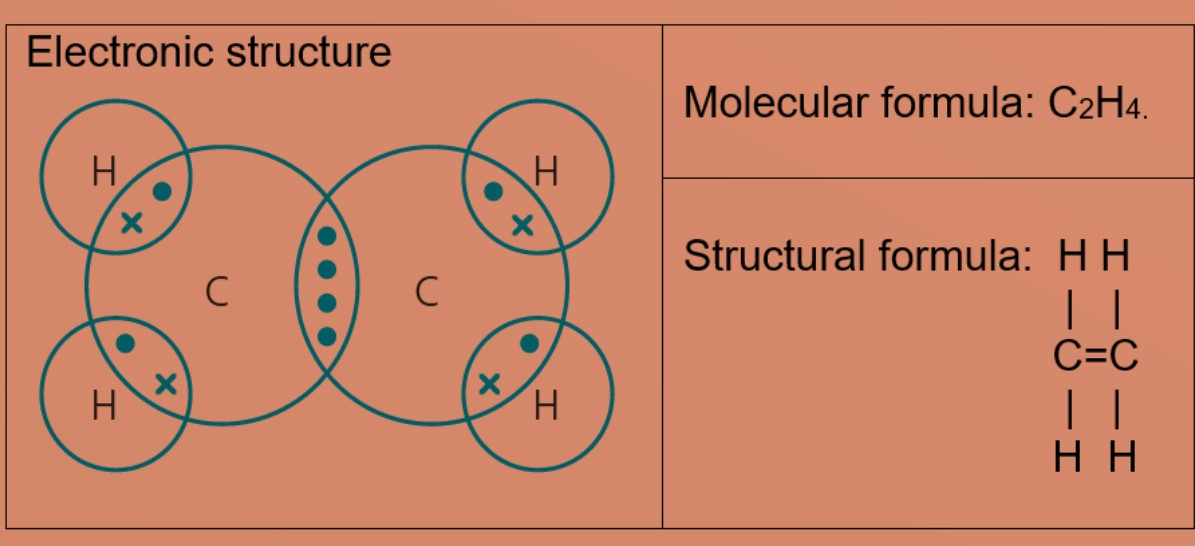
Eg 2: Ethene
A carbon atom has four valence electrons, it needs four more electrons to be stable.
Hydrogen atom needs one more electron to be stable and carbon atoms will share 2 valence electrons with 2 hydrogen atoms. After sharing 2 valence electrons with hydrogen atoms to form single covalent bond, each carbon atom now needs two more valence electrons and share two valence electrons with each other to form double covalent bonds between two carbon atoms. This results in hydrogen atoms having 2 valence electrons in first shell and carbon has eight valence electrons.
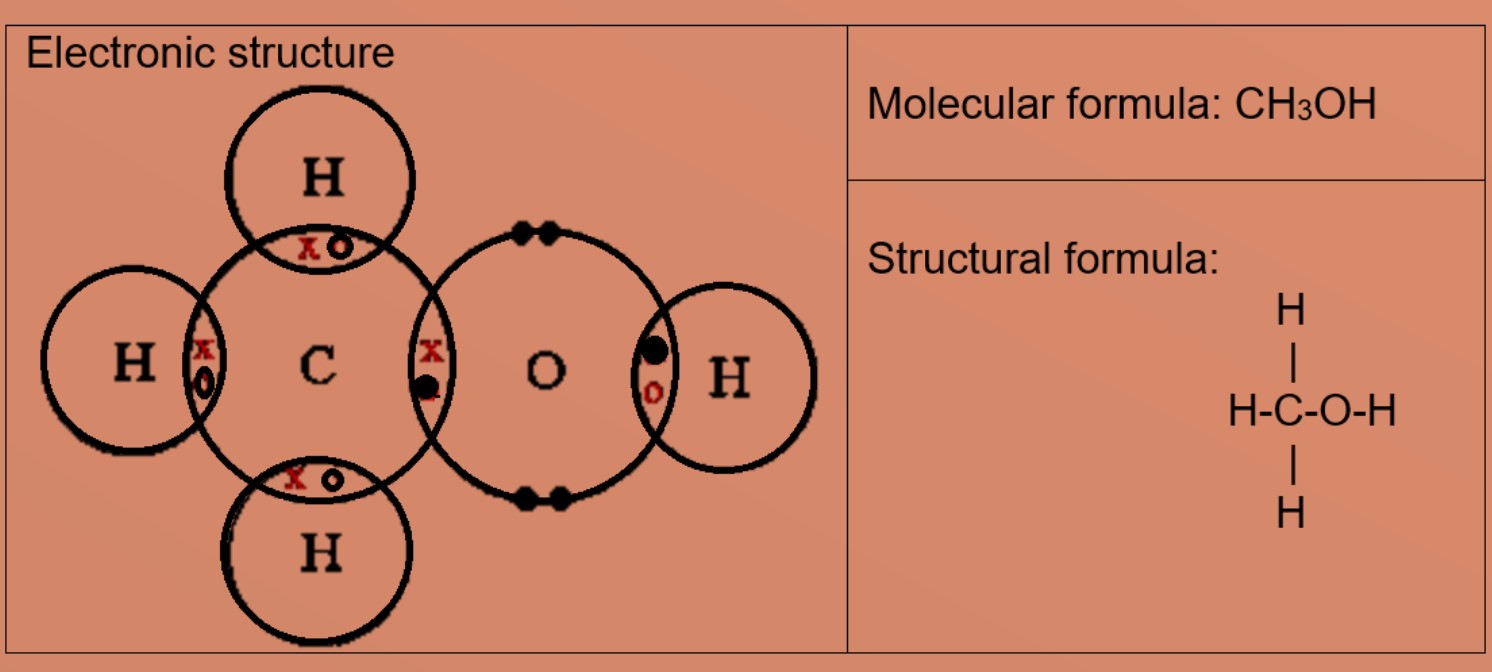
Eg3: Methanol
A carbon atom has four valence electrons, it needs four more electrons to be stable. Oxygen atom has 6 valence electrons and needs 2 more valence electrons to be stable. The carbon atom shares 3 valence electrons with three hydrogen atoms and 1 valence electron with one oxygen atom. Oxygen atom will share 2 valence electrons, one with carbon and one with hydrogen. This results in hydrogen atoms having 2 valence electrons in first shell and carbon and oxygen has eight valence electrons each.
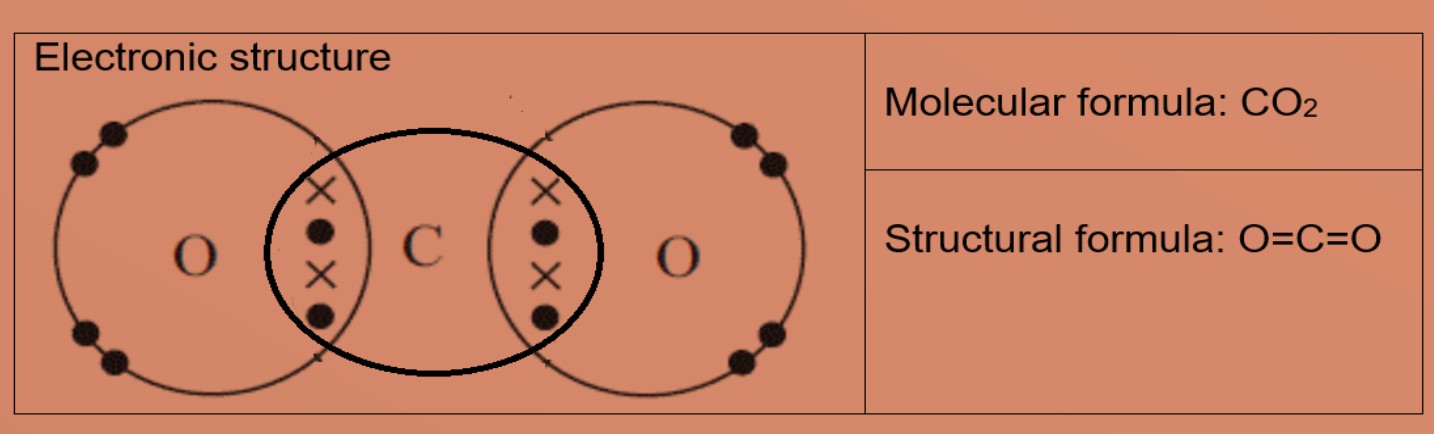
Eg4: Carbon dioxide
A carbon atom has four valence electrons, it needs 4 more electrons to be stable. Oxygen atom has 6 valence electrons and needs 2 more valence electrons to be stable. The carbon atom shares all four valence electrons: two with each oxygen atom. Each oxygen atom will share 2 valence electrons with carbon atom, form 2 double covalent bonds in the molecule. This results in carbon and oxygen has eight valence electrons each.
• Explain the differences in melting point and boiling point of ionic and covalent compounds in terms of attractive forces
Ionic compounds have high melting and boiling points due giant ionic lattice structure with strong ionic bonds (electrostatic forces of attraction between positively charged ions). Large amount of energy is needed to break these strong bonds.
Covalent compounds usually have low melting and boiling point due to simple molecular structure with weak intermolecular forces. Small amount of energy is needed to overcome these weak forces.